Avoiding burnout of white blood cells
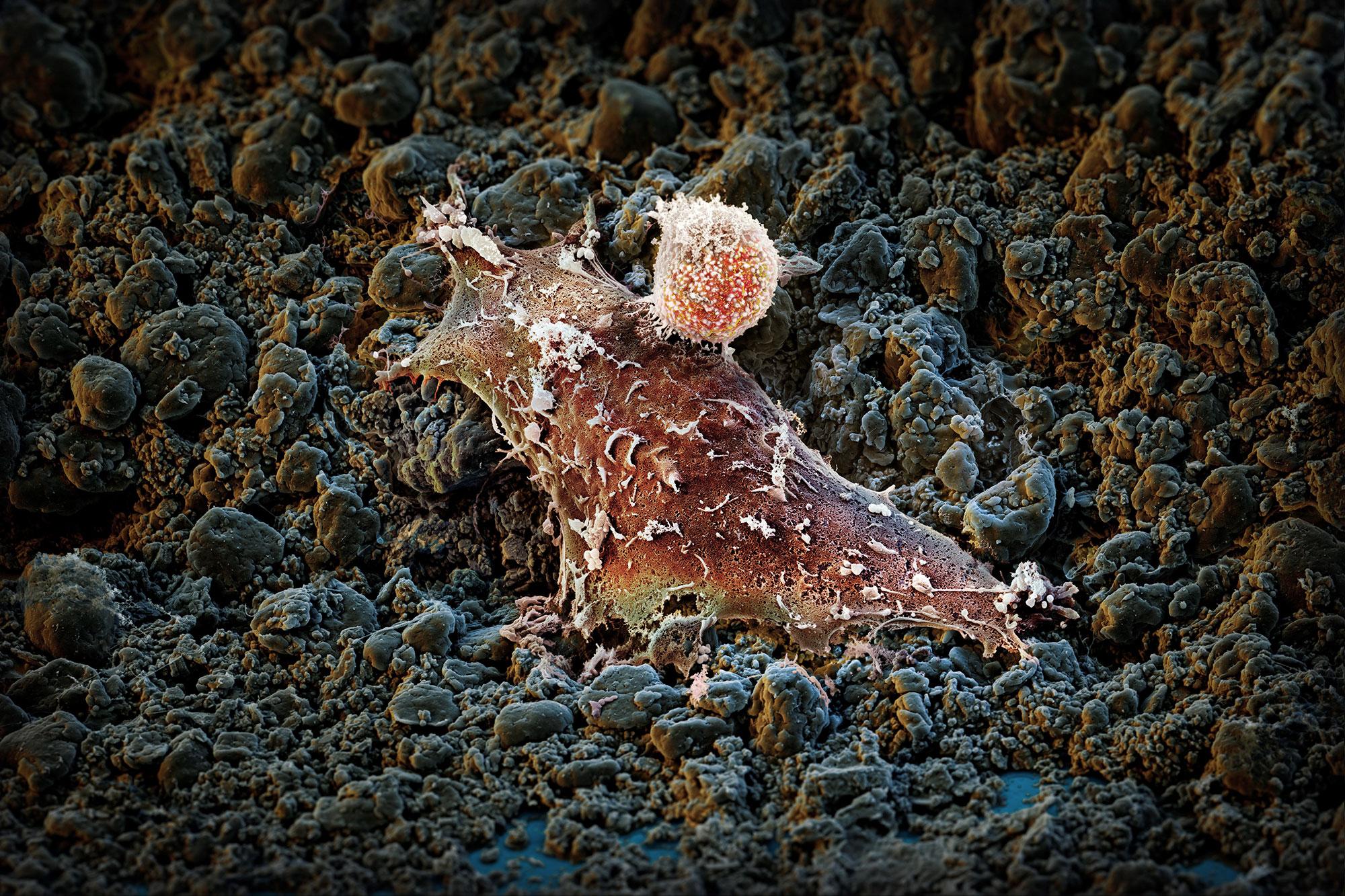
A research group funded by the Swiss National Science Foundation has identified a gene that drives T lymphocytes to exhaustion. This opens up new approaches for more effective immunotherapies.
A tough battle requires endurance. This is also true for white blood cells as they tackle cancer – or more specifically for T lymphocytes or T cells, a group of white blood cells involved in the immune system’s fight against cancer cells. However, T cells can become exhausted during this fight. Researchers from the Department of Biomedicine at the University of Basel and University Hospital Basel recently identified a gene that seems to contribute to this exhaustion. The findings of their research project, which was funded by the Swiss National Science Foundation, were published in the journal Nature Communications (*).
T lymphocyte exhaustion has been a known problem for around 20 years. After a chronic exposure to tumour cells, T cells enter a state of exhaustion and become less effective: while they continue to recognise the hostile tumour cells, they produce fewer substances to eliminate them. In addition, they can no longer develop into memory T cells, which are important in supporting the immune response. This exhaustion therefore also impacts on the effectiveness of immunotherapies, which are based on stimulating the body’s own immune defences against cancer cells. “This also applies to cell therapies to tackle cancer: even if ‘new’ T cells are injected into patients, the exhaustion remains a problem,” explains Alfred Zippelius, co-author of the study.
Fine-tuning
The research group therefore tried to better understand the mechanisms leading to T cell exhaustion. They developed a model based on human tumor cells and produced exhausted lymphocytes, similar to those found in the tumours of patients.
The team then examined a variety of genes by inactivating them individually using the CRISPR/Cas9 method. This enabled them to identify a gene regulating T cell exhaustion. T cells remain functional when this gene – known as SNX9 – is inactivated, even when they are in the vicinity of a tumour over a longer period. “The SNX9 gene seems to increase the short-term immune response, which can prove important in situations in which every hour counts to tackle a disease. In the case of our experiment, however, suppressing the SNX9 gene enabled finer adjustment of immune cell activity by reducing excessive stimulation signals. The T cells’ activity was therefore conserved over a longer period,” explains Marcel Trefny, lead author of the study. The study also found that rather than simply dying after doing their job, the T cells developed into memory T cells more frequently. “The discovery of the role of this gene opens up new approaches for more efficient immunotherapies,” summarises Alfred Zippelius.
These findings are very promising, as there is a lack of targets to prevent T cell exhaustion and most experiments to characterise such targets have been performed in mouse cells. However, the therapeutic application of this new approach must now be clinically tested to find out whether the absence of this gene can cause adverse effects.
Funding research in all disciplines
This research work was supported by the SNSF through the project funding scheme. Following a selection process, researchers can use these grants to carry out projects with topics and goals of their own choice.
Links
- Image for editorial use. Caption (JPEG): The immune system (T cell) attacks a human tumor cell. © M. Oeggerli (Micronaut 2019), Marcel Philipp Trefny, and Prof. Alfred Zippelius, Translational Oncology, University Hospital Basel, supported by Pathology University Hospital Basel, and C-CINA, Biozentrum, University Basel, (Das Bild darf nur zur Illustration der Arbeit von Marcel Trefny und Alfred Zippelius verwendet werden.)
- The project on the SNSF Data Portal
- SNSF Twitter feed